Más Información
Turistas visitan Puerto Vallarta tras jornada violenta por operativo contra "El Mencho"; pasean si preocupación, afirman

Velocidad de fake news superan en 1000% esfuerzos de verificación, señala experta; terror digital también es una estrategia del narco

PVEM se deslinda de Pedro Segura Valladares tras amenazas a Loret; "no es ni ha sido militante del Partido Verde", asegura

Bajo fuerte dispositivo de seguridad, Diócesis de Apatzingán realiza procesión por la paz en Aguililla, Michoacán; es la cuna de "El Mencho"
Mexico’s Foreign Affair’s Ministry Marcelo Ebrard
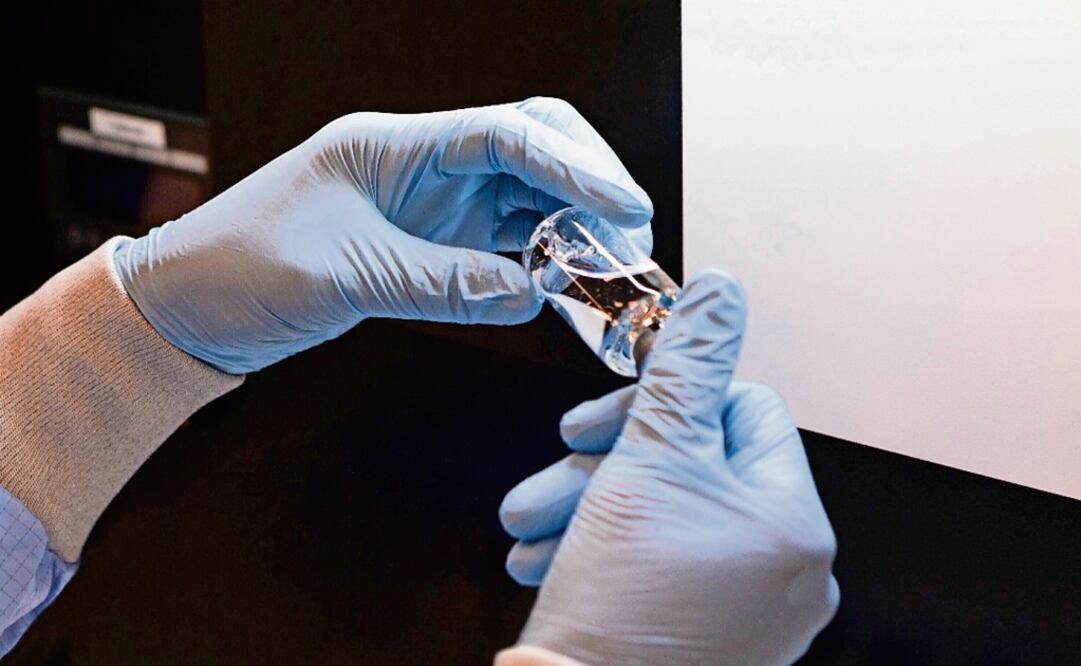
asserted that seven patients infected with COVID-19 at the National Institute of Nutrition are partaking in a clinical trial with a U.S. laboratory on the use of remdesivir as a cure for the new disease.
In President López Obrador’s morning news conference at the National Palace, Ebrard said that the number of patients will increase in the second stage .

Ebrard mentioned that the Mexican government is waiting for the agreement with the laboratory of the World Health Organization to determine the total number of patients that will be included in the next protocol that will have a larger scope.
Recommended: Mexico approves clinical trials for COVID-19 drugs
By late March, deputy Health minister Hugo López-Gatell said that the Federal Commission for the Protection Against Health Risks (COFEPRIS) had approved three out of four upcoming clinical trials for COVID-19 drugs.
Last April 20, Ebrard said via Twitter that he had been in a videoconference, along with Health Minister Dr. Jorge Alcocer Varela , with members from the Gilead laboratory that “presented progress on the clinical trials of the remdesivir medication for possible COVID-19 treatments.”
After Jorge Alcocer informed that the first clinical trials on the use of remdesivir that were approved for Mexican patients with serious COVID-19 cases had shown encouraging results, López-Gatell asked the population to remember it should not be used as other drugs we already know.
“ Remdesivir is not a drug approved for general use, neither in Mexico nor in any part of the world; we must not expect to find a drug soon. I’m enthusiastic, just as the Health minister, but it’s not a drug for general use,” he insisted.
He explained that there have been results during the pandemic, nevertheless, people must not expect its general use to be approved soon. “They are modest results ; I say it because it’s completely valid for the population to be hopeful for a drug to be used for the treatment of their relatives, however, it’s important for the population to clearly understand the status of this kind of research.”
López-Gatell said that thinking about remdesivir as a drug for general use could be detrimental .
“It’ wouldn’t be unusual for there to be pressure from different fronts to purchase the drug or for suppliers to cause someone to want to sell it; we must not be mistaken, its use is only approved for clinical trials .”
Recommended: Lessons from the past: What we've learned from the history of pandemics
mp
Noticias según tus intereses
[Publicidad]
[Publicidad]